 |
| Figure 1. Growth curves of the sheep on the low, medium and high nutrient intakes |
| Livestock Research for Rural Development 23 (5) 2011 | Notes to Authors | LRRD Newsletter | Citation of this paper |
The study investigated the reliability of using faecal progesterone as an indicator of ovarian activity in Sabi ewes and how this is affected by level of feeding. Three diets were formulated to simulate feed availability in communal areas of Zimbabwe, a low nutrient diet, a medium nutrient diet and a high nutrient diet. Faecal and blood samples were collected from 18 ewes (six in each treatment) once every two days for two oestrus cycles in order to determine progesterone levels.
Diet had a significant effect on the plasma progesterone concentration (P<0.05) and a non significant effect on faecal progesterone concentration (P>0.05). On all diets, faecal progesterone concentrations were higher than plasma progesterone concentration. The pattern of faecal and plasma progesterone concentration were positively correlated in ewes on a medium nutrient diet (r=0.920, P<0.05) than on a low nutrient diet and a high nutrient diet (r=0.6881, P>0.05 and r=0.50, P>0.05). Response-relationship between faecal and plasma progesterone concentration profiles was non-linear (R-square=0.25, P>0.05) for ewes on a high nutrient diet and linear for a low nutrient diet and a medium nutrient diet (R-square=0.46, P<0.05 and R-square=0.84, P<0.05). Faecal progesterone can be used as an alternative method of monitoring the ovarian activity in ruminant animals on a medium nutrient level of feeding.
Keywords: Faecal progesterone, maintenance diet, ovarian activity, plasma progesterone, Sabi ewes
Small ruminant production by resource-poor farmers in the
communal areas of Zimbabwe is limited by low reproductive rates. Inadequate
nutrition is one of the major limiting factor and main contributor to poor
reproduction (Clatworthy 1998). Currently, the resource-poor farmers are
striving to increase the number of livestock they keep in order to uplift their
life styles. There is need for a cost effective method for monitoring the
reproductive performance especially ovarian function by small holder farming
communities where basic facilities for collection, processing and storage from
research animals are not available.
Progesterone is a steroid hormone secreted mainly by cells of the corpus luteum, placenta and adrenalin gland. In sheep the length of a normal oestrus cycle is 17 days, the corpus luteum attains full secretory activity between the sixth and eighth day and continues to secrete progesterone at a constant rate until day 15. Thereafter secretion declines until the next oestrus (Cunnigham et al 1975). This decline is associated with the abrupt termination of the functionality of the corpus luteum that occurs at the end of the luteal phase (Gordon 1997). The regression of the corpus luteum occurs only if fertilisation does not occur. If fertilization occurs after ovulation the corpus luteum does not regress and the concentration of progesterone in the blood remains elevated through out pregnancy (Gordon 1997). In ewes progesterone secreted by the corpus luteum has many functions before and after fertilization. Exogenous progesterone administration improved embryonic survival in animals at risk of abortion (Parr et al 1987). Serum progesterone has also been used to check the effectiveness of ovulation induction. Luteinizing hormone stimulates glandular production of progesterone in the uterus, which then facilitates implantation of the embryo and maintenance of pregnancy. Progesterone influences udder development (Hamudikuwanda 1998) by complete development of the alveoli of the mammary gland.
Quantity and quality of natural grazing varies with season (Nyamukanza et al 2010). This affects productive and reproductive performance of livestock. (Clatworthy 1998). Nutritional factors can influence the hypothalamus-pituitary function and therefore gonadotrophin profiles, directly through effects of nutrients or metabolic hormones such as insulin acting on targets organs or through changes in sensitivity of these organs to oestradiol, progesterone and other hormonal feedback (Rhind 1992).
In cows, ovarian activity can be monitored by measuring concentration of circulating steroid hormones in blood and faeces (Masunda et al 1998). Shemesh et al (1979) showed that it is possible to measure progesterone concentration in milk and relate levels to ovarian function in ewes but only to lactating ewes. Urine samples can also be collected to monitor ovarian activity (Evans et al 1984) but animals must be in metabolic cages. Progesterone can be measured in faeces irregardless of the animal’s physiological status. Most studies concentrated on faecal progesterone measurement in relation to ovarian activity and pregnancy (Desauliners et al 1989; Masunda et al 1988). There are no reports on the level of feeding on oestrus induced fluctuations of faecal progesterone concentration. This information is useful for timing sample collection and predicting the reproductive status of animals under range conditions where availability and quality of feeds is highly variable with seasons.
It is imperative to find effective and appropriate techniques to monitor ovarian function of small ruminant animals using basic equipment. This will provide information on possible means of manipulating reproduction and improving reproduction rates. The study aims to find whether faecal progesterone can be used as a reliable indicator of ovarian activity in ewes and investigate the effect of protein and energy levels of feeding on faecal progesterone.
The study was carried in an animal house located at the department of Animal Science, University of Zimbabwe.
Eighteen non-pregnant, non lactating Sabi ewes which were known to have shown regular oestrus cycle prior to the experiment were used in this study. Two weeks prior to the start of the experiment oestrous was synchronised in the ewes by administering two injections (11 days apart) of a synthetic prostaglandin, Estrumate®. Five millilitres were injected subcutaneously into each animal. Synchronization of oestrus was done to enable the simultaneous collection of blood and faecal samples at different stages of oestrus cycle. The animals were put in metabolism crates designed to separate faeces and urine and allow each animal to be fed individually. Fresh water was always available. The animals were fed ad-libitum fresh diets every morning after the removal of the refusal from the previous meal. Animals were weighed weekly and feed intake recorded daily. Faecal and feed samples collected were then used to calculate the digestibility of the treatment diet at the end of the trial. Digestibility was calculated as the difference between the DM (dry matter) percentage of feed and faecal samples divided by feed sample DM. Weight gain of the animals was also calculated.
Three experimental diets which simulated seasonal changes in feed availability and quality (protein and energy) in communal areas were fed as shown in Table 1. Sheep concentrate is high energy and protein formulated animal feed. Treatment L (low) contained 90% Katambora hay and 10% sheep concentrate, which supplied 0.5 times energy and protein required for maintenance of ewes (low nutrient level). It simulated feed available during peak dry season (early July to late September). Treatment M (medium) supplied a medium nutrient level of feed, which simulated availability during the start of the rainy season (early October to late November and then late April to late June). The diet contained 35% sheep concentrate and 65% Katambora hay. Treatment L (high) diet contained 12.5% Katambora hay and 87.5% sheep concentrate which supplied 1.5 times the protein and energy required by the ewe for maintenance (high nutrient level). It resembled feed availability in communal areas during peak of the rain season (late November to late March).
|
Table 1. Physical and Chemical composition of diets |
|||
|
Treatments |
|||
|
L |
M |
H |
|
|
Ingredients |
|
|
|
|
Katambora Hay (%) |
90.0 |
65.0 |
12.5 |
|
Sheep concentrate (%) |
10.0 |
35.0 |
87.5 |
|
Chemical composition |
|
|
|
|
DM (%) |
89.0 |
89.0 |
90.0 |
|
CP (%DM) |
5.44 |
7.54 |
11.2 |
|
CF(%DM) |
34.1 |
27.5 |
13.9 |
|
EE (%DM) |
5.45 |
5.08 |
4.28 |
|
ME (MJ/kg DM) |
9.01 |
10.7 |
14.0 |
|
L for low nutrient status, M for medium and H is high nutrient status |
|||
A completely randomised design (CRD) was used. The animals were stratified into weight groups and then from each group there were randomly allocated to the treatment groups such that each group had six animals.
Blood and faecal samples were collected from each ewe once every two days for two oestrous cycles. Sampling was done between 0800 and 1000hrs. The first collection of samples was done two days after the administration of the second estrumate injection. During collection, ten millilitres of blood was collected by jugular vein puncture and placed into tubes containing EDTA (anticoagulant factor). The samples were immediately chilled in iced water at 40C .The blood samples were centrifuged at 2000g for 15 minutes within 1 hour of collection. The separated plasma was stored at -200C subsequent progesterone assays.
Fresh faeces, which dropped into the collection trays during sampling time, for each ewe, were collected and dried in an oven at 600C for 48 hours. Thereafter, they were ground with a Wiley grinding mill passing through a 3mm screen size. The samples were then stored at room temperature for subsequent progesterone analysis.
Progesterone assays were extracted from faecal samples using a protocol described by Desauliners et al (1989) with a few modifications. A sample of 200-250mg of dried and ground faeces was weighed and put in a test tube. Distilled water (2ml) was added and the mixture was extracted three times using 3ml diethyl ether at each extraction. During extraction, the mixture was vortexed for 3 minutes. The extracted sample was put in a freezer overnight at -200c to allow the separation of the organic and aqueous phases. The organic phase was decanted into 10ml glass tubes and left to evaporate under a fume chamber. The residue was dissolved in 1ml of ethanol and then diluted a further 100-times using an assay buffer (0.1M sodium phosphate dibasic, 0.9% sodium chloride, 0.1% sodium azide and 0.1% gelatine) of Ph 7.0 before radio immunoassay were performed.
During the assay for progesterone, a direct solid phase radio-immuno assay method using antibody coated tubes and I125Progestrone (coat-a-Count Kits, Diagnostic Products, Corporation, Los Angeles, USA) were used to determine the plasma and faecal progesterone concentration.
Pearson’s correlation coefficient between plasma progesterone and faecal progesterone concentrations throughout the oestrus cycle was computed using PROC CORR procedure of the Statistical analysis system. A t- test for the difference in live weight gain was computed. A PROC GLM procedure was used to calculate the mean, standard error and maximum and minimum progesterone concentrations in plasma and faecal samples. Faeces progesterone concentration was regressed against plasma concentration. Co-variation analysis for difference in initial mean live weight was also considered. Results of the regression analysis were used to formulate equations which explain the cause response relationship between plasma and faecal progesterone concentration.
DM digestibilities of the three treatment diets showed significant differences (P<0.05). Treatment L, M and H had 43, 57, and 72% DM digestibility, respectively. As expected, the diets differed in CF, CP, and ME contents (see Table 1). The highest percentage of CF was observed in treatment L and lowest in treatment H. There was no significant differences in the fat content (P>0.05) as shown in Table 1.
There was a significant difference in DM intake (P<0.01) of the treatment diets. Animals that were on low nutrient diet (treatment L) consumed an average of 0.65kgDM/day. This low average intake resulted in a corresponding low intake of the feed nutrients in the diets (i.e. CP, CF, ME, and Fat) as shown in Table 2. Animals on a medium nutrient diet (treatment M) and a high nutrient diet (treatment L) consumed an average of 1.75kgDM and 1.53kgDM of feed per day, respectively. This consequently increases the intake of CP and ME per day. The intake of fats was not significantly different among the three treatments (see Table 2).
|
Table 2. Feed intake and live weight gain in association with DM digestibility of the treatment diets |
|||||
|
|
Nutrient density |
SEM |
P |
||
|
Low |
Medium |
High |
|||
|
DM digestibility, % |
43a |
57b |
72c |
0.927 |
<.001 |
|
Daily feed intake |
|
|
|||
|
DMI, kg/day |
0.65a |
1.16b |
1.53c |
1.34 |
<0.01 |
|
CP, g |
35.5a |
87.2b |
172 |
1.86 |
<.001 |
|
ME, MJ/kg DM |
58.8a |
123b |
214c |
4.79 |
<.001 |
|
CF, g |
223a |
318b |
212c |
3.92 |
<.001 |
|
Fat, g |
35.6a |
58.8a |
41.8a |
2.91 |
0.061 |
|
ME is calculated as 0.15x TDN (AFRC technical committee on response to nutrients 1993) a,b, Row means with different superscripts differ (P <0.05) |
|||||
Table 3 and Figure 1 summarize the effect of three treatments on live weight. Treatment diets showed a significant effect on live weight gain (P<0.01). Ewes on treatment L showed a significant difference in the initial and final weight which resulted in ewes losing an average of 189g/day whilst those on treatment M maintained their weight throughout the trial with non-significant live weight gain of 15g/day. A continuous significant change in live weight in ewes on treatment L was observed and the ewes gained weight at a rate of 160g/day.
|
Table 3. Change in live weight of ewes during the trial |
|||||
| Treatment |
SEM |
P |
|||
|
Low |
Medium |
High |
|||
|
Initial mean weight, kg
|
33.5a (0.84) |
34.2a (0.64) |
33.4a (0.96) |
0.697 |
0.683 |
|
Mean live weight per week, kg |
|
|
|
|
|
|
1 |
30.1b |
35.1a |
34.0ab |
0.282 |
<.001 |
|
2 |
27.3b |
35.0a |
36.0ab |
0.484 |
<.001 |
|
3 |
26.5b |
35.3a |
38.0ab |
0.443 |
<.001 |
|
4 |
25.4b |
34.3a |
36.0ab |
0.441 |
<.001 |
|
5 |
25.2b |
32.8a |
39.3ab |
0.421 |
<.001 |
|
Final mean live weight, kg |
24.4b |
35.3b |
41.1b |
0.282 |
<.001 |
|
SEM |
0.580 |
0.846 |
0.486 |
|
|
|
P |
<0.001 |
0.024 |
<0.001 |
|
|
|
Live weight change, g/day |
-189a |
15b |
160c |
0.00587 |
<.001 |
|
a,b, Row means with different superscripts differ (P <0.05); |
|||||
 |
| Figure 1. Growth curves of the sheep on the low, medium and high nutrient intakes |
Diet and day had a significant effect on plasma progesterone concentration profile (diet, P< 0.05 and day, P< 0.05) as illustrated in Figure 2. There was no significant effect of day and diet during post ovulatory period (Day 1 to day 4, P>0.05). From day 6 to 18, a significant effect was observed with highest progesterone concentration profiles in treatment L and lowest in treatment L. The last two days of the oestrus cycle showed no significant effect of day and diet on plasma progesterone concentration profile. The trend of the profile remained the same across the treatments. There was no change in progesterone concentration in ewes on treatment L (P>0.01) during the oestrus cycle. The difference in the concentration across treatments was highly significant between day 6 and day 18 (P<0.01)
 |
|
Figure 2. Mean concentration of progesterone in plasma of ewes exposed to different levels of feeding during the oestrous cycle |
A significant effect of day on faecal progesterone concentration (P<0.05) was observed especially after day 4 of the oestrus cycle as shown in Figure 3. However, the effect of diet could not be separated (P>0.05). There was no significant effect of day on faecal progesterone concentration in ewes on treatment L during post ovulatory period (day 1 to day 8). There after, an elevation in progesterone concentration was observed until day 12. A sharp decline in the concentration was evident for ewes on treatment L and 3 on the 14th day of oestrus cycle. Faecal progesterone concentration of ewes on treatment L and 2 were observed to be following the same trend with higher concentrations from day 1 to day 11 as compared to those on treatment L. From day 4 to day 8, ewes on treatment L had the highest concentration as compared to their counter-parts in treatment M and 3.
 |
| Figure 3. Mean faecal progesterone concentration of ewes exposed to different levels of feeding during the oestrous cycle |
Figures 4, 5 and 6 show the trend of plasma and faecal progesterone concentration in ewes exposed to treatments 1, 2 and 3, respectively. The concentration of faecal and plasma progesterone in ewes on treatment M were correlated (P<0.05; r=0.92) and a similar relationship was found in ewes on treatment L (P<0.05; r=0.68). There was no correlation between faecal and plasma progesterone concentration in ewes on treatment L (r=0.50, P>0.05).
 |
| Figure 4. Average concentration of progesterone in plasma and faeces of ewes fed a low nutrient diet |
 |
| Figure 5. Mean concentration of progesterone in plasma and faeces in sabi ewes fed a maintenance diet |
Equations showing plasma and faecal progesterone concentration cause response are shown below,
For treatment L
Y=0.02+0.26X (R-square=0.46, P<0.05)
For treatment M
Y=0.02+0.57X (R-square=0.85, P<0.05)
For treatment L
Y=0.82+0.45X (R-square=0.25, P>0.05)
Where: Y= plasma progesterone concentration (ng/ml)
X=faecal progesterone concentration (x1000ng/g)
The regression coefficient (0.56) and R-square (0.85) for ewes on treatment M were greater than those on treatments 1 and 3 as indicated in the equations above.
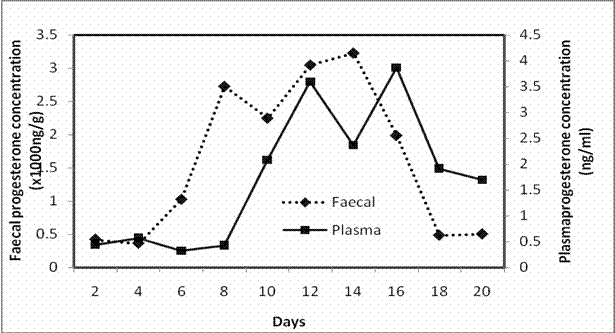 |
| Figure 6. Average concentration of progesterone in plasma and faeces of sabi ewes fed a diet above maintenance |
The pattern of plasma progesterone levels observed during the oestrus cycle was similar to that previously reported for ewes (Cunningham et al 1975). The profile, however, shows that diet had an effect on the ovulation rates reflected by the difference in plasma progesterone concentration in ewes exposed to different treatment diets. Ewes subjected to chronic underfeeding show suppressed oestrous.
The faecal progesterone concentrations obtained in this study are within the range reported for Holstein heifers (Desauliners et al 1989, Larter et al 1994). Catabolised progesterone in the liver is passed through bile into the digestive tract and end up in faeces as conjugated steroids. The concentration of progesterone is affected by the level of dietary fibre ( Wasser et al 1994). The authors noted that dietary fibre increase gastro-intestinal (GI) transit time, while increasing faecal bulk and total faecal progesterone. This is attributed to the reduced rate of passage and could be an explanation for the profile of faecal progesterone concentrations observed in ewes on treatment L (day 4-8). The reduced rate of passage allowed the accumulation of progesterone in the GIT which then tend to elevate the concentrations in faeces. Earlier observations by Wasser et al (1993) proposed that relatively high circulating basal concentrations of progesterone result in a persistently high baseline excretion rate in faeces regardless of dynamic changes in plasma progesterone concentration. This explains why a significant and non-significant change in progesterone concentration in faeces and faeces and plasma, respectively was detected at the same time in ewes on treatment L.
There was a long lag-time (of about 4 days) between progesterone secretion in the blood and excretion in faeces (excretion lag-time), thus resulting in the progesterone secretion profiles being unreliable for informing the physiological events occurring in the ovaries. This could be attributed to low CF percentage and high digestibility of the diet which shortens the storage time of the faeces in the gastro-intestinal tract (GIT). In ewes on treatment M, the lag time was almost zero with a correlation close to one (r=0.92, P<0.05). This proves the rationale behind the use of faecal progesterone as an alternative method of monitoring the activity in animals on a maintenance diet. This observation was, however, not applicable for ewes on treatment L and 2.
The amount of variation in plasma progesterone that can be accounted for the collection of faeces for progesterone was highest in ewes on treatment M (R-square=0.85) and lowest in ewes on treatment L (R-square=0.25). Treatment L showed that the relationship of plasma and faecal concentration do not fit in a linear model distribution (P>0.05). Treatment L and 2 fit very well in the model (p<0.05).
There are two limitations associated with the use of faecal progesterone for monitoring physiological events in the ovaries of ruminant animals, especially in communal areas. Firstly, the time-course of progesterone excretion in faeces relative to biological events in the ovaries for animals during late November to late March (high nutrient level of feed) is long and secondly, faecal progesterone concentration mis-inform the ovarian activities in animals during the period from early July to late September (low nutrient feed level). The concentration of progesterone in the faeces exaggerates the ovarian activities in the ruminant animals.
AFRC 1993 Technical Committee on Responses to Nutrients Energy and Protein Requirements of Ruminants. CAB International.
Clatworthy J N 1998 Veld: The Basic Feed. Cattle producers association. Beef production manual CPA 98.6
Cunningham N F, Symous A M and Saba N 1975 Levels of progesterone, L.H and F.S.H in the plasma of sheep during oestrus cycle. Journal of reproduction and fertility 45:177-180
Desauliners D M, Goft A K, Bettridge K J, Rowell J E and Flood P F 1989 Reproductive hormone concentration in faeces during the oestrus cycle and pregnancy in cattle (Bos taurus) and musk oven (Obivious moschetus). Canadian journal of zoology 67: 1148-1154
Evans K L, Kasman L H and Hughes J P 1984 Pregnancy diagnosis in the domestic horse through direct urinary progesterone conjugate analysis. Therogenology 22: 615-620.
Gordon I 1997 Contolled reproduction in sheep and goats. Controlled reproduction in farm animals series, Volume 2, CAB International.
Hamudikuwanda H 1998 Fertility in the beef herd. Cattle producers’ association. Beef production manual 98/13
Larter N C, Rajamahendran R and Sivakumaran 1994 The use of total immune-reactive faecal progestagens to monitor reproductive function in cattle. Veterinary record 134: 474-475
Masunda B, Mutisi C and Hamudikuwanda H 1998 The use of faecal progesterone measurement to monitor ovarian function in Zimbabwean cattle. U.Z/RVAU/DANIDA project review workshop held at University of Zimbabwe
Nyamukanza C C, Scogings P F, Mbatha K R and Kunene N W 2010 Forage-sheep relationships in communally managed moist thornveld in Zululand KwaZulu-Natal South Africa. African Journal of Range and Forage Science 27:11-19.
Parr R A, Davis I F, Faridough R J and Miles M A 1987 Overfeeding during early pregnancy reduces peripheral progesterone concentration and pregnancy rate in Sheep. Journal of Reproduction and Fertility 80:317-320
Shemesh M Ayalon N and Mazor T 1979 Early pregnancy diagnosis in ewes based on milk progesterone levels Journal of Reproduction and Fertility 56: 301-304
Rhind S M 1992 Nutrition: It’s effects on reproductive performance and it’s hormonal control in female sheep and goats. In: Progress in Sheep and Goats Research. CAB International, Wallingford.
Wasser S K, Monfort S L, Southers J and Wildt D 1994 Excretion rates and metabolites of oestradiol and progesterone in Baboon ( Papio cynocephalus ) faeces. Journal of Reproduction and fertility 101:213-220
Wasser S K, Thomas R, Nair P P, Guidry C, Monfort S L, Southers J and Wildt D E 1993 Effect of dietary fibre on faecal steroid measurements I baboons (Papio cynocephalus) faeces. Journal of Reproduction and fertility 97: 569-574.
Received 9 February 2011; Accepted 24 March 2011; Published 1 May 2011