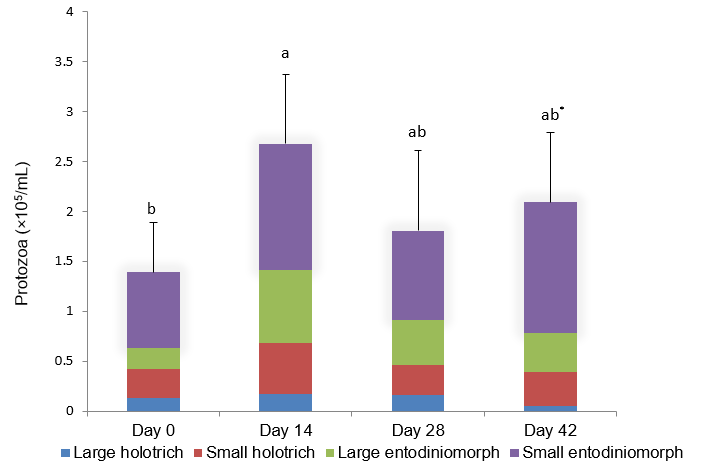
error bars indicate s.d. of the total number and common superscripts above error bars indicate non-significant differences;
* protozoal concentrations from strained rumen fluid through double layer of cheese cloth at slaughter on d 45.
| Livestock Research for Rural Development 31 (9) 2019 | Guide for preparation of papers | LRRD Newsletter | Citation of this paper |
This study aimed to quantify resident ciliate protozoal populations both in the digesta and on the surface of the reticulum, rumen and omasum of Angus cattle. Ruminal, reticular and omasal contents and tissues were collected from Angus heifers (n = 8; 226 ± 11.8 kg; 8 months of age) at slaughter to determine the total protozoal population and its distribution within different parts of cattle foregut both in the digesta and on the surface. Angus heifers were previously offered ad lib lucerne cereal hay mix and no feed being offered on the day at slaughter. Protozoa were counted using a Fuchs–Rosenthal optic counting chamber and were differentiated into large (>100 µm) and small (<100 µm) holotrich and entodiniomorph groupings. The majority of protozoa at slaughter (>99%) were present in free digesta not adhering to the gut wall (<1.0%). The populations in the reticular and omasal digesta, while much smaller than (~5% of) that in the rumen, were of similar size to each other. The omasum surface, however, provides sequestration for a similar number of protozoa than does the entire rumen surface, indicating that the omasum may be an important reservoir for protozoa, especially entodiniomorphs. This is a likely reason why removal of rumen protozoa in cattle is difficult due to omasal contents containing residual of omasal protozoa re-inoculated the defaunated rumen.
Keywords: cattle, defaunation, foregut, protozoa
Removal of protozoa from the rumen (defaunation) increases bacterial biomass and increases flow of protein into the duodenum, which is associated with an increase in growth rate of defaunated relative to faunated ruminants (Bird and Leng 1978; Eugène et al 2004; Jouany 1996). Defaunation can also decrease enteric methane production (Hegarty 1999; Morgavi et al 2008, Nguyen et al 2016) by eliminating methanogens that exist as endo- and ecto-symbionts with ciliate protozoa (Finlay and Esteban 2013) and by changing the molar proportions of VFA to a greater proportion of propionate and lesser proportion of butyrate (Eugène et al 2004). A recent meta-analysis by Newbold et al (2015) reported that removal of ciliate protozoa from the rumen of ruminants increased livestock average daily gain (ADG) by 9% and reduced enteric CH4 emissions by 11%. The positive effect of defaunation on growth rate is often reported when the feed is deficient in protein relative to energy content (Eugène et al 2004 ; Williams and Coleman 1992). However, Towne and Nagaraja (1990) found that the residual omasal protozoa would re-inoculate the rumen of the defaunated rumen, and this would explain why few studies of defaunation were reported to completely render ruminants without ciliate protozoa. This study, therefore, aimed to quantify resident ciliate protozoa populations both in the digesta and on the surface of the reticulum, rumen and omasum of Angus cattle.
All protocols for treatment and care of the cattle were approved by the University of New England Animal Ethics Committee (AEC 14-117). Angus heifers (n = 8; 226 ± 11.8 kg; 8 months of age) were offered 12 kg/head/day of a chaffed lucerne cereal hay mix (9.7 MJ ME/kg DM and 14.3% CP, Table 1) with the feed offered twice daily in two equal portions.
Samples of rumen fluid were collected every 2 weeks from each heifer before feeding using oesophageal intubation for protozoal enumeration from d 0 to d 42. On d 45, heifers were transported by truck 30 min to a local abattoir and killed the same morning with no feed being offered. Immediately after slaughter and evisceration, the reticulum and omasum were located and tied off to avoid flow of digesta within the foregut. The complete reticulum, rumen and omasum with and without digesta were weighed individually to determine weights of digesta contained and tissue weight of each organ. Digesta from each organ was thoroughly mixed and approximately 20 g sub-samples were collected in 25 mL open ended syringes with the tops cut off. The liquid was then squeezed out by placing a doubled layer of cheese-cloth over the open end then pushing the plunger into the barrel of the syringe. The pH of the resulting strained liquid (liquid fraction) was immediately measured using a portable pH meter (Orion 230 Aplus, Thermo scientific, Beverly, MA, USA) and then the liquid samples were preserved in pre-weighed containers containing formaldehyde-saline (4% formalin v/v; 0.9% NaCl w/v). The particulate digesta retained on the cheese-cloth (solid fraction) was also preserved in pre-weighed containers containing formaldehyde-saline. The containers were later re-weighed to determine weights of liquid and solid samples. Gut tissue samples were cut from each organ where the locations were identically located in each animal. Samples of gut tissues were fixed on plastic boards (3.5×4.5 cm) and then the samples were gently rinsed in clean water to wash out the digesta before preservation in pre-weighed containers with 10% (v/v) formalin to enable adherent protozoa to be counted. The containers later were re-weighed to determine the weight of gut tissue preserved.
|
Table 1. Chemical composition of the lucerne cereal hay mix (g/100g DM) |
|
|
Component |
Lucerne cereal hay mix |
|
Dry matter (in feed as-fed) |
88.7 |
|
Dry matter digestibility |
69 |
|
Digestible organic matter |
67 |
|
Organic matter |
90 |
|
Neutral detergent fibre |
42 |
|
Acid detergent fibre |
31 |
|
Crude protein |
14.3 |
|
Crude fat |
1.4 |
|
Metabolisable energy (MJ/kg DM) |
9.7 |
A subsample (1.0 mL) of preserved liquid fraction was pipetted into a test tube. Two drops (0.05 mL) of brilliant green (2.0 g of brilliant green dye and 2.0 mL of glacial acetic acid diluted to 100 mL with distilled water) were added (Dehority 1984). The contents were mixed and allowed to stand overnight before counting of protozoal cells by microscopy.
A portion of each preserved sample of the digesta ‘solid’ fraction was further diluted with formaldehyde-saline, vortexed and then sonicated for 5 min to remove adherent protozoa. The homogenized preserved ‘solids’ samples were squeezed out through a doubled layer of cheese-cloth to separate liquid and solid fractions. A subsample (1.0 mL) of liquid was pipetted into a test tube to be stained with brilliant green. The solid content was placed in a 25 mL beaker to be thoroughly mixed and then 1.0 g of subsample was placed in a test tube. Three drops (0.075 mL) of brilliant green were added and vortexed to ensure thorough mixing. The contents were allowed to stand overnight. The preserved gut tissue samples were placed in 50 mL beaker, thoroughly mixed and sonicated for 15 min to release adherent protozoa from the tissue. A 1.0 mL sample of sonicated preserved liquid fraction was pipetted into a test tube to be stained with brilliant green and allowed to stand overnight.
After staining, a portion of each stained sample was diluted with 30% glycerol, resulting in 1:20 dilution of the original sample. Protozoa were counted using a Fuchs–Rosenthal optic counting chamber (0.0625 mm 2 and 0.2 mm of depth) using a staining technique adapted from the procedure described by Dehority (1984). The protozoa were differentiated into large (>100 µm) and small (<100 µm) holotrich and entodiniomorph groupings. Total protozoal populations in, or on an organ’s surface, were estimated as the product of weight of digesta (or weight of tissue) times the protozoa/g of digesta or protozoa/g of rinsed gut tissue for that organ.
Data were subject to analysis of variance (PROC GLM) using SAS 9.0 (SAS Instute, Cary, NC). Protozoa counts were log-transformed to meet homogeneity of variance and normal distribution criteria using PROC UNIVARIATE before statistical analysis. Least significant differences were used for means separation (p < 0.05).
Rumen protozoal concentrations (cells/mL) monitored over 42 days prior to slaughter (Figure 1) showed protozoal concentrations had increased after initial introduction to the mixed lucerne and cereal chaff diet (a 48% increased over first 14 days; 1.39×105 v 2.68×105; p < 0.05). There were no significant differences in protozoal concentrations from d14 to d 42 (p > 0.05), indicating that concentrations of rumen protozoa were stable in the weeks leading up to slaughter. Protozoal concentrations from the strained rumen fluid at slaughter on d 45 were similar to that of samples collected on d 0, 14, 28 and 42 from oesophageal intubation (Figure 1; p > 0.05). Small entodiniomorphs were dominant in the rumen fluid, ranging from 47 to 65% of the total protozoa (Figure 1).
 |
| Figure 1.
Protozoal population densities (cells/mL) in rumen fluid from Angus
heifers receiving lucerne cereal hay mix over a period of 42 days; error bars indicate s.d. of the total number and common superscripts above error bars indicate non-significant differences; * protozoal concentrations from strained rumen fluid through double layer of cheese cloth at slaughter on d 45. |
The pH of digesta did not differ between reticulum, rumen and omasum. The rumen had the largest mass of digesta of all the forestomachs (Table 2; p < 0.05). The total number of protozoa in the rumen was higher than in the reticulum and omasum. Although the quantity of digesta in the reticulum was smaller than in the omasum, the total number of protozoa was similar in both reticulum and omasum.
Weight of gut tissue was the greatest for the rumen followed by the omasum and reticulum (Table 2; p < 0.05). However, the total population of protozoa in rumen was not different from in the omasum. High numbers of entodiniomorphs were found in the omasum, but holotrichs were not detected.
|
Table 2. Ciliate protozoa in reticular, ruminal and omasal contents and gut tissues of Angus heifers |
|||||
|
Parameter |
Mean (n=8) |
Pooled |
p |
||
|
Reticulum |
Rumen |
Omasum |
|||
|
pH |
6.29 |
6.38 |
6.28 |
0.13 |
0.83 |
|
Digesta weight in grams |
905c |
34,113a |
4,540b |
678 |
<0.001 |
|
Total protozoa (×106) |
147.35b |
7,015a |
188.35b |
1.32 |
0.01 |
|
Large holotrichs |
29.81b |
594.67a |
14.82b |
1.46 |
<0.001 |
|
Small holotrichs |
60.40b |
775.11a |
15.67c |
1.44 |
<0.001 |
|
Large entodiniomorphs |
19.43c |
1,967a |
46.81b |
1.28 |
<0.001 |
|
Small entodiniomorphs |
37.71c |
3,678a |
111.05b |
1.45 |
<0.001 |
|
Gut tissue weight in grams |
1,111c |
6,446a |
4,901b |
245.3 |
<0.001 |
|
Total protozoa (×105) |
0.71b |
5.85a |
4.58a |
0.28 |
0.003 |
|
Large holotrichs |
0.15b |
1.01a |
- |
0.49 |
0.01 |
|
Small holotrichs |
0.33b |
2.38a |
- |
0.36 |
0.003 |
|
Large entodiniomorphs |
0.12b |
1.62a |
2.27a |
0.26 |
<0.001 |
|
Small entodiniomorphs |
0.11b |
0.84ab |
2.31a |
0.28 |
<0.001 |
|
Different superscripts indicate significant difference within rows |
|||||
Protozoal concentrations vary among animals and are dependent on many factors such as ruminant species, geographical location (Akbar et al 2009), diets (Whitelaw et al 1984), frequency of feeding (Williams 1986) and rumen pH (Clarke 1977). The protozoal populations in rumen fluid after straining through a double layer of cheese-cloth to remove large plant fibres were similar to those of samples collected from oesophageal intubation (Figure 1; p > 0.05), indicating that experiments involve in sampling rumen fluid from oesophageal intubation for protozoal enumeration may offer a precise estimate of protozoal populations being free in the liquid fraction of the rumen contents. This normal method of enumeration does not account for the protozoa adhering to plant particles (Bauchop and Clarke 1976), these authors found high concentrations of rumen protozoa attaching on the damaged surface of plant fragments, between layers of plant cells and among vessel elements with the protozoa being identified as the entodiniomorphs: Epidinium ecaudatum; Eudiplodinium spp., Diplodium spp. and the holotrich Dasytricha spp. Hook et al (2012) also reported a majority (63 - 90%) of rumen protozoa existed in the attached phase, either on the feed particles or in the rumen wall.
Ciliate protozoa contribute disproportionately little to the nitrogen nutrition of ruminants with protozoal nitrogen being up to 53.4% of total microbial biomass in the rumen (Michałowski 1979) but only 20% of total microbial nitrogen entering to the duodenum (Jouany et al 1988). The smaller protozoal biomass in the duodenum of ruminants could reflect 65% and 74% of protozoa die and are degraded in the rumen of sheep and cattle, respectively (Ffoulkes and Leng 1988; Leng 1982), suggesting that only 24 - 35% of rumen protozoa enter to the lower digestive tract, while the majority of rumen protozoa are retained and lyse within the rumen. Further, the protozoal biomass leaving the rumen is greater than that arriving in the duodenum because of some rumen protozoa being trapped in the omasal leaves (Czerkawski 1987). The relatively high numbers of protozoa found in the omasum in this study may explain that the lower protozoal contribution to the total microbial nitrogen outflow in the duodenum is partly due to high numbers of protozoa being retained in the omasum of ruminants.
Ruminants with protozoa in the rumen (faunated) support a higher ruminal NH3 concentration than do animals with rumen protozoa removed (defaunated), indicating that rumen protozoa degrade dietary proteins (Jouany 1996) and engulf bacteria for their amino acid requirement (Esteban et al 2014). Elimination of rumen protozoa increases bacterial biomass and increases growth rate of ruminants (Newbold et al 2015) especially when feed is deficient in protein rather than energy content. This leads to some circumstances in which rumen protozoa may limit animal productivity. However, existing procedures to eliminate ciliate protozoa involve dosing ruminal contents with antiprotozoal detergents, but these methods of defaunation are not always successful. In sheep, defaunation had been successfully with sodium 1-(2-sulfonatooxyethoxy) dodecane (Nguyen et al 2016), but chemical defaunating detergents are not often reported successful in rendering cattle free of ciliate protozoa for prolonged period (Nguyen and Hegarty 2017). Towne and Nagaraja (1990) completely eliminated rumen protozoa of Holstein steers by emptying ruminal contents, flushing the omasum and spraying 1 L of dioctyl sodium sulfosuccinate solution on rumino-reticulum walls and on the reticulo-omasal orifice. However, the authors found live protozoa in the rumen contents a day after the treatment.
The flow of digesta from the reticulum occurs following the omasal canal contractions, but occasionally backflow of large volumes of digesta from the omasum to the reticulum occurs when the omasal body contracts during the closure of omaso-abomasal orifice (Stevens et al 1960). Therefore, Towne and Nagaraja (1990) claimed that the backflow of omasal contents containing residual of omasal protozoa reinoculated the defaunated rumen of steers. That could explain why defaunation of cattle is difficult as residual omasal protozoa may be responsible for the reappearance of rumen protozoa after ruminal defaunation is complete.
The relatively large total populations of ciliate protozoa in the digesta and rumen wall of cattle indicate that the total populations of protozoa in the rumen cannot be simply counted by the conventional enumeration, although concentrations of rumen protozoa in the rumen fluid were similar in samples collected by oesophageal intubation and by slaughter. This study confirms there is a large reservoir of protozoa in the omasum of cattle and it is proposed their backflow into the rumen contributes to reducing the effectiveness of chemical defaunation of the rumen. Techniques that completely eliminate protozoa from the omasum as well as the rumen of cattle will be required if the advantages of increasing the microbial nitrogen outflow and efficiency of feed utilization by elimination of rumen protozoa are to be realized in the cattle industries.
The authors are grateful for the assistance of Dr Mark Barnett and Mr Graeme Bremner, University of New England, with technical supports and sample collection. This study was sponsored by the University of New England, DVCR International Fee Scholarship and by Vietnam International Education Development.
Akbar T, Ali M S, Golamreza Z, Maghsoud B and Adel A 2009 The study of diversity of ciliate protozoa in Ghizel sheep fed in pasture and nourished by dried grape by-product. American Journal of Animal and Veterinary Sciences 4(2): 37-41. doi:10.3844/ajavsp.2009.37.41
Bauchop T and Clarke R T 1976 Attachment of the ciliate Epidinium Crawley to plant fragments in the sheep rumen. Applied and Environmental Microbiology 32(3): 417-422.
Bird S G and Leng R A 1978 The effects of defaunation of the rumen on the growth of cattle on low protein high-energy diets. British Journal of Nutrition 40: 163-167. doi: 10.1079/bjn19780108
Clarke R T J 1977 Protozoa in the rumen ecosystem. In ‘Microbial ecology of the gut’ (Ed. Clarke T T J and Bauchop T), pp. 251-275. New York: Academic Press.
Czerkawski J W 1987 Reassessment of the contribution of protozoa to the microbial protein supply to the host ruminant animal. Journal of Theoretical Biology, 126(3), 335-341. https://doi.org/10.1016/S0022-5193(87)80240-0
Dehority B A 1984 Evaluation of subsampling and fixation procedure used for counting rumen protozoa. Applied and environmental microbiology 48(1): 182-185.
Esteban G F, Finlay B J and Warren A 2014 Free-Living Protozoa. In 'Ecology and General Biology.' (Ed. Thorp JH and Rogers DC) Vol. 1 pp. 113-132. Academic Press: Boston.
Eugène M, Archimède H and Sauvant D 2004 Quantitative meta-analysis on the effects of defaunation of the rumen on growth, intake and digestion in ruminants. Livestock Production Science 85: 81-97. https://doi.org/10.1016/S0301-6226(03)00117-9
Ffoulkes D and Leng R A 1988 Dynamics of protozoa in the rumen of cattle. British Journal of Nutrition, 59, 429-436. doi: 10.1079/bjn19880051
Finlay B J and Esteban G F 2013 Protozoa. In 'Encyclopedia of Biodiversity.' (Ed. Finlay B J) pp. 286-297. Academic Press: Waltham.
Hegarty R S 1999 Reducing rumen methane emissions through elimination of rumen protozoa. Australian Journal of Agricultural Research 50(8): 1321-1328. https://doi.org/10.1071/AR99008
Hook S E, Dijkstra J, Wright A D G, McBride B W and France J 2012 Modeling the distribution of ciliate protozoa in the reticulo-rumen using linear programming. Journal of Dairy Science 95(1): 255-265. doi:http://dx.doi.org/10.3168/jds.2011-4352.
Jouany J P 1996 Effect of rumen protozoa on nitrogen utilization by ruminants. The Journal of Nutrition, 126: 12-1335. doi: 10.1093/jn/126.suppl_4.1335S
Jouany J P, Demeyer D I and Grain J 1988 Effect of defaunating the rumen. Animal Feed Science and Technology, 21(2–4): 229-265. https://doi.org/10.1016/0377-8401(88)90105-8
Leng R A 1982 Dynamics of protozoa in the rumen of sheep. British Journal of Nutrition 48(2): 399-415. https://doi.org/10.1079/BJN19820123
Michałowski T 1979 Effect of feeding frequency on the diurnal changes in microbial protein, volatile fatty acids and ammonia contents of the rumen of sheep. The Journal of Agricultural Science 93(1): 67-70. https://doi.org/10.1017/S0021859600086135
Morgavi D P, Jouany J P and Martin C 2008 Changes in methane emission and rumen fermentation parameters induced by refaunation in sheep. Australian Journal of Experimental Agriculture 48(2): 69-72. https://doi.org/10.1071/EA07236
Newbold C J, de la Fuente G, Belanche A, Ramos-Morales E and McEwan N 2015 The role of ciliate protozoa in the rumen. Frontiers in Microbiology, 6. https://doi.org/10.3389/fmicb.2015.01313
Nguyen S H and Hegarty R S 2017 Effects of defaunation and dietary coconut oil distillate on fermentation, digesta kinetics and methane production of Brahman heifers. Journal of Animal Physiology and Animal Nutrition 101(5): 984-993. doi: 10.1111/jpn.12534. Epub 2016 Jul 6.
Nguyen S H, Bremner G, Cameron M and Hegarty R S 2016 Methane emissions, ruminal characteristics and nitrogen utilisation changes after refaunation of protozoa-free sheep. Small Ruminant Research 144: 48-55. https://doi.org/10.1016/j.smallrumres.2016.08.002
Stevens C E, Sellers A F and Spurrell F A 1960 Function of the bovine omasum in ingesta transfer. American Journal of Physiology 198: 449-455. 10.1152/ajplegacy.1960.198.2.449
Towne G and Nagaraja T G 1990 Omasal ciliated protozoa in cattle, bison, and sheep. Applied and environmental microbiology 56(2): 409 - 412.
Whitelaw F G, Eadie J M, Bruce L A and Shand W J 1984 Methane formation in faunated and ciliate-free cattle and its relationship with rumen volatile fatty acid proportions. British Journal of Nutrition 52(2): 261-275. doi: 10.1079/bjn19840094
Williams A G 1986 Rumen holotrich ciliate protozoa. Microbiological Reviews 50(1): 25-49. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC373052/
Williams A G and Coleman G S 1992 The rumen protozoa. Springer-Verlag: New York, NY, USA.
Received 5 March 2019; Accepted 22 August 2019; Published 1 September 2019