

of sweet and bitter varieties of cassava
of sweet and bitter varieties of cassava
| Livestock Research for Rural Development 31 (1) 2019 | Guide for preparation of papers | LRRD Newsletter | Citation of this paper |
This experiment aimed to measure N retention and rumen methane production when goats had access to mixed sweet and bitter varieties of cassava foliage (SW-BI) compared with the sweet variety alone (SW). In each feeding system, biochar (1% of diet DM) and brewers’ grains (4% of diet DM) were supplied singly or in combination or not at all. Eight Bach Thao goats (LW 16.4 ± 2.03 kg) were allocated to a double 4*4 Latin square design, with one square for each of the main effects of cassava variety. The four combinations of additives were the treatments within each of the Latin squares.
Levels of condensed tannins were similar in leaves of sweet and bitter varieties of cassava and were higher in leaves than in petioles. Leaves of the bitter variety had 72% more precursors of HCN than leaves of the sweet variety. HCN precursors were higher in leaves than in petioles.
The goats with free access to both cassava foliage varieties (BI-SW) consumed equal parts of each, but the overall intake of DM was 25% higher and the N retention was 23% higher compared with feeding the sweet variety alone. N retention was increased when either brewers’ grains or biochar or both were added to the diet. The combined effect of mixed cassava foliage with brewers’ grains and biochar represented a 58% increase in N retention compared with sweet cassava alone and no additives. Rumen ammonia and methane were lower when the goats had access to both sweet and bitter cassava foliage. The reduction in rumen methane was positively correlated with N retention. Dietary treatments had no effect on the pattern of rumen VFA .
It is proposed that HCN precursors present in higher concentrations in the leaves of bitter compared with sweet cassava leaves induced a partial shift in digestion of nutrients from the rumen to the lower digestive tract facilitating more efficient use of dietary protein by enzymic digestion in the small intestine and reducing the formation of methane which is not produced when fermentation takes place in the cecum-colon.
Key words: condensed tannins, hydrocyanic acid (HCN), rumen escape of nutrients, tannins
In an experiment in our laboratory with cattle fed foliages from known “sweet” (Gon) and bitter (KM94) varieties, as protein-rich supplements to ensiled cassava pulp-urea (Binh et al 2017), there were major differences in animal response with reduced feed intake and negligible growth rate (61 g/d) when the bitter cassava was fed compared with normal feed intake and growth rate (383 g/d) when the cassava foliage was from the sweet Gon variety. The important observation in that experiment was that the addition of a low level of brewers’ grains (4% of diet DM) to the “bitter” cassava diet led to similar feed intakes and growth rates as for cattle fed the sweet cassava foliage. It was hypothesized that the low-level addition of brewers’ grains was acting as a “prebiotic” in facilitating the detoxification of the HCN produced in the cattle fed the bitter cassava foliage. This was confirmed by analysis of the urine taken before and after addition of brewer’s grains to the diet which showed a 50 % reduction in thiocyanate concentrations in the urine from 90 to 55 ppm.
In a follow-up to this experiment, the “prebiotic” effect of a low level of brewers’ grains was evaluated in Cambodia with growing goats fed a 100% cassava foliage diet (Sina et al 2017). Growth rates were more than doubled (from 71 to 161 g/day) when the cassava foliage was supplemented with brewers’ grains at the rate of 5% of their DM intake. The variety of cassava was unknown but was assumed to be of a “sweet” variety commonly grown in Cambodia for human consumption. The increased growth rate resulted from a combination of an increase in DM intake (47%) and in DM digestibility (29%). The concentration of thiocyanate in urine was reduced from 28 to 11.5ppm when brewers grains were added to the diet confirming that part of the effect of the supplement was to aid in the detoxification of hydrocyanic acid (HCN) produced in the animal from enzymic conversion of the cyanogenic glucosides present in cassava foliage in varying concentrations depending on variety (Phuong et al 2015).
Increased understanding of the role of prebiotics as support for biofilms and their associated microbial communities involved in the animal’s digestive system led to an appraisal of the potential role of biochar as a prebiotic, following it’s known ameliorating properties in soils (Lehmann 2007; Preston 2015) considered to be due to its interactive role in supporting microbial communities in this medium.
In an initial study with a cassava-based diet fed to local Yellow cattle in Laos (Leng et al 2012), growth rates were increased, and the methane content of eructed gas was reduced when 1% biochar was added to the diet. More recent studies have shown: (i) synergistic effects from combining biochar with rice distillers’ byproduct as additives to a cassava-based diet for fattening cattle (Sengsouly and Preston 2016); and (ii) that biochar added to a basal diet of urea-treated cassava stems fed to goats increased daily N retention by 46% and the biological value of the absorbed N by 12% (Thuy Hang et al 2018).
The aim of the following experiment was to test the individual and combined effects of biochar and brewers’ grains as “prebiotics” in goats fed diets of 100% cassava foliage. Contrasting intakes of cyanogenic glucosides were achieved by feeding: (i) foliage of a sweet variety as the sole diet; or (ii) allowing the goats to have free access to foliage of both sweet and bitter varieties.
The experiment was conducted in Nong Lam University, Ho Chi Minh city, Viet Nam, from May to August 2018.
Eight male Bach Thao goats were allocated to two 4*4 Latin Square designs (Table 1). In the first Latin Square, the goats were fed “sweet” cassava foliage (Gon variety) ad libitum as basal diet. In the second Latin Square, the goats had free access to foliage of both sweet (Gon) and bitter cassava (KM94) varieties. In each square there were 4 combinations of “additives”. Each period lasted 23 days; 18 days for adaptation to the treatments followed by collection of feces and urine over the last 5 days.
The treatments in each square were:
CTL: No additive
BG: Brewer’s grain (at 4% of diet DM)
Bio: Biochar (1% of diet DM) suspended in 30 g molasses to prevent loss of particulate matter
BGBio: The combination of brewers’ grains and biochar.
|
Table 1. Layout of each Latin Square |
||||
|
Period/goat |
1 |
2 |
3 |
4 |
|
1 |
CTL |
Bio |
BGBio |
BG |
|
2 |
BG |
CTL |
Bio |
BGBio |
|
3 |
Bio |
Bio |
BG |
CTL |
|
4 |
BGBio |
BG |
CTL |
Bio |
Bitter cassava foliage was grown in a farmers’ field 30 km distant from the University while the sweet cassava foliage was on a farm 20km distant. Total time for bitter foliage collection in the field, transportation and feed preparation was 2 h including: (i) spending 15-20 minutes to collect 4-6 kg fresh foliage and removing the stems followed by: (ii) 1.5h for transportation by bus; and (iii) 15 minutes for weighing the foliage according to the experimental diets. For sweet cassava foliage the overall time was 1.5h.
Brewer’s grains were purchased from the beer factory located in Ho Chi Minh city at 3-day intervals and stored in woven polypropylene bags during the 3 days. Molasses and biochar were prepared at the beginning of the experiment and stored for use over the whole experimental period. Biochar was made in an updraft biochar stove using rice husks as fuel.
The eight male Bach Thao goats had an average initial weight 16.4 ± 2.03 kg. They were housed in raised cages with facilities for separate collection of urine and feces. During a 10-day period prior to starting the experiment, they were: vaccinated against endemic diseases and treated for parasites by subcutaneous injection of Ivermectin; adapted to eating the cassava foliage. In both square 1 and square 2, sweet cassava foliage (tender stems with petioles and leaves attached) was suspended above the feed trough (to simulate browsing). In square 2, after 5 days with only the sweet variety, the bitter variety was introduced and gradually increased until the goats had free access to both the sweet and bitter varieties.
The procedure during the actual experiment was:
Day 1 to 14: The additives were offered according to the designated treatments. Adaptation to the additives was by mixing a small amount of cassava leaves with the additive and then removing gradually the leaves over 4-5 days until the goats were seen to directly consume the additive (Biochar was always mixed with molasses in ratio of 1:5 by weight).
Day 15; Samples of rumen fluid were taken by stomach rube 2 hours after first feeding for measurement of ammonia and molar proportions of volatile fatty acids (VFA). The pH was measured with a digital meter and sulphuric acid added as preservative prior to analysis of VFA.
Day 16: A sample of ample of urine was taken for determination of thiocyanate
Day 17: A closed chamber was used for methane measurement. After a 10 minute period to take a ”blank” sample of air the goats were introduced to the chamber for ten minutes for their breath to equate with the air in the chamber. This was followed by successive measurements over 20 minutes of the concentrations of methane and carbon dioxide in mixed air and eructed gas. The procedure was that described by Madsen et al (2010), using an infra-red gas meter (GASMET 4030; Gasmet Technologies Oy, Pulttitie 8A, FI-00880 Helsinki, Finland).
Day 18: Live weights were recorded in the morning prior to the first feeding.
Days 19-23: Sulphuric acid (10% concentration) was added daily to the urine container (at 10% of total volume) to keep the pH under 4. The daily amounts of urine and feces were collected and weighed, and the feces frozen at -100C. At the end of each collection period: the daily amounts of feces were thawed and mixed thoroughly to provide a representative sample for analysis; (ii) daily collections of urine were mixed and sub-samples taken for analysis.
Samples of feed offered, feed residues and feces were dried to constant weight at 650C to prepare them for analysis. DM% was then determined heating at 100 0C to constant weight. Total nitrogen content was determined by Kjeldahl method (AOAC 1990). Condensed tannin in cassava leaves and petioles was determined by boiling the samples in 0.1N H 2SO4, adding indigo dye and titrating with potassium permanganate according to AOAC (2016) official method 955.35. Cyanide as equivalent hydrogen cyanic acid content (HCN) in fresh cassava leave was determined as per AOAC (2016), official method 915.03B. Thiocyanate was determined following the procedure described in the protocol of kit D1 ( http://biology-assets.anu.edu.au/hosted_sites/CCDN/five.html). Ammonia in rumen fluid was measured by a colorimetric method using UV spectrophotometric detector at wavelength of 640 nm. Determination of molar proportions of volatile fatty acids (VFA) was by high performance liquid chromatography (HPLC).
The data for DM intake and N retention were analyzed using the Repeated Measures option in the General Linear Model in the ANOVA program of the SAS Software (SAS 2010). The repeated measures were the daily DM intakes, and daily quantities of DM and N in feces and of N in urine during days 21-25 of the collection period. Other data were analyzed by the General Linear Model option in the ANOVA program of the Minitab Software (Minitab 2016). In each model the sources of variation were: variety of foliage, additives, interaction foliage*additives and residual error.
Levels of condensed tannins were similar in leaves of sweet and bitter varieties of cassava and were higher in leaves than in petioles (Table 2; Figure 1). Leaves of the bitter variety had 72% more precursors of HCN than leaves of the sweet variety (Figure 2). HCN precursors were higher in leaves than in petioles.
|
Table 2. Mean values (DM basis except % DM which is on fresh basis) for effects of cassava foliage (sweet or bitter) on % DM, tannin and HCN equivalent in leaves and petioles |
||||||
|
Leaf |
Petiole |
p |
SEM |
|||
|
Bitter |
Sweet |
Bitter |
Sweet |
|||
|
DM, % |
28.7 a |
26.4 a |
15.6 b |
16.4 b |
0.004 |
2.24 |
|
Tannin, % |
3.18a |
2.98 ab |
2.13 ab |
1.9 b |
0.022 |
0.288 |
|
HCN, mg/kg |
1282 a |
745 b |
296 c |
415 c |
<0.001 |
65.1 |
 |
 |
| Figure 1.
Condensed tannin in petiole and leaf from foliage of sweet and bitter varieties of cassava |
Figure 2.
HCN equivalent in petiole and leaf from foliage of sweet and bitter varieties of cassava |
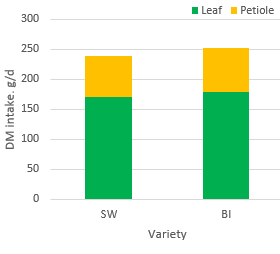
When the goats had access to foliage from both sweet and bitter cassava, they consumed equal parts of each (Table 3; Figure 3).
|
Table 3.
Mean intakes of leaf and petiole (g DM /d) for the goats in |
||
|
Sweet |
Bitter |
|
|
Leaf |
171 |
179 |
|
Petiole |
67.2 |
73.2 |
 |
 |
 |
| Figure 3. DM intake of leaf and petiole of sweet and bitter cassava varieties when the goats had free access to both in Square 2 | Figure 4. Effect of cassava variety on DM intake | Figure 5. Effect of additives on DM intake |
Overall intake of DM was 25% higher for the offer of mixed varieties compared with the sweet variety alone (Table 3; Figure 4). DM intake was higher when both brewers grains and biochar were added to the diet compared with the additives given separately or were not given (Figure 5). There was no interaction between source of cassava foliage and the source of additives.
Coefficients of apparent digestibility of dry matter and crude protein did not differ between goats fed mixed sweet and bitter foliage compared with those fed only the sweet variety (Table 4).
|
Table 4. Mean values (g/d) for effects of cassava foliage (bitter or sweet ) and of additives (none, biochar, brewers’ grains or both) on DM intake (DMI), apparent digestibility of DM and crude protein (CP) and N balance |
||||||||||
|
Cassava variety |
SEM |
p |
Additives |
SEM |
p |
|||||
|
Bitter+sweet |
Sweet |
None |
Bio |
BG |
BGBio |
|||||
|
DMI |
496 |
408 |
8.35 |
<0.0001 |
449 a |
436 a |
441 a |
482 b |
8.35 |
0.0002 |
|
Apparent digestibility, % |
||||||||||
|
DM |
74.7 |
74.4 |
0.804 |
0.79 |
75.5 |
73.3 |
74.7 |
74.6 |
1.14 |
0.58 |
|
CP |
81.9 |
81.2 |
0.563 |
0.43 |
82.8 |
80.3 |
81.7 |
81.4 |
0.796 |
0.19 |
|
N balance, g/d |
||||||||||
|
Intake |
19.1 |
15.3 |
0.39 |
<0.001 |
15.4 |
14.4 |
15.3 |
16 |
0.46 |
0.108 |
|
Feces |
3.51 |
2.71 |
0.10 |
<0.001 |
2.91 |
3.11 |
3.13 |
3.29 |
0.137 |
0.28 |
|
Urine |
5.85 |
4.57 |
0.27 |
<0.001 |
6.31b |
4.38a |
5.58b |
4.56a |
0.376 |
<0.001 |
|
Retention |
8.68 |
7.14 |
0.45 |
<0.0001 |
7.13a |
7.81a |
7.58a |
9.12b |
0.449 |
0.0032 |
|
ab Means within main effects without common superscript differ at p=0.05 |
||||||||||
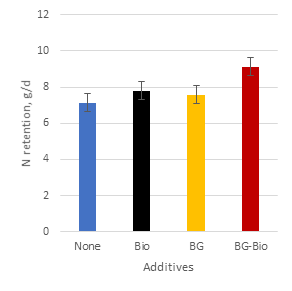
Effects of cassava variety on nitrogen retention mirrored those on DM intake with an overall increase of 25% when the mixed cassava varieties were offered (Figure 6). N retention was increased when either brewers’ grains or biochar (Table 4; Figure 7) or both (Figure 8) were added to the diet. The combined effect of mixed cassava foliage with brewers’ grains and biochar (Figure 9) represented a 58% increase in N retention compared with sweet cassava alone and no additives.
|
Table 5. Mean values for effects of additives on N retention (g/d) |
||||||
|
NoBG |
BG |
p |
Nobio |
Bio |
p |
SEM |
|
6.53 |
7.37 |
0.07 |
6.36 |
7.54 |
0.011 |
0.325 |
 |
 |
| Figure 6. Effect of cassava variety on N retention | Figure 7. Main effects of brewers’ grains (4% of diet DM) and biochar (1% of diet DM) on N retention |
 |
 |
| Figure 8. Individual and combined effects of brewers’ grains and biochar on N retention |
Figure 9. Individual and combined effects of additives and source of cassava foliage; (SW Sweet: SW-BIT Sweet and Bitter) on N retention |
Rumen ammonia was lower when the goats had access to both bitter and sweet cassava foliage compared with access only to foliage from the sweet variety (Table 6). There were no treatment effects on the pattern of the rumen VFA.
|
Table 6. Mean values for effects of cassava foliage (bitter or sweet ) and of additives (none, biochar, brewers’ grains or both) on VFA proportions (mol %), acetic:propionic ratio, rumen ammonia, daily urine volume, daily excretion of thiocyanate (SCN) in urine and CH4:CO2 ratio in mixed eructed gas and air |
||||||||||
|
Cassava foliage |
SEM |
p |
Additives |
SEM |
p |
|||||
|
SW |
SW-BI |
None |
Bio |
BG |
BG-Bio |
|||||
|
Ac |
81.1 |
77.3 |
3.21 |
0.42 |
76.1 |
75.5 |
83.2 |
82.1 |
4.54 |
0.52 |
|
Pr |
9.53 |
11.5 |
1.35 |
0.31 |
10.9 |
11.8 |
9.81 |
9.57 |
1.91 |
0.83 |
|
Bu |
9.37 |
11.1 |
2.93 |
0.68 |
13.0 |
12.7 |
7.01 |
8.31 |
4.14 |
0.66 |
|
Ac:Pr |
9.26 |
9.00 |
1.11 |
0.86 |
9.53 |
7.59 |
9.31 |
10.1 |
1.57 |
0.71 |
|
NH3, mg/l |
514 |
423 |
30.0 |
0.042 |
396 |
459 |
535 |
484 |
42 |
0.17 |
|
Urine, ml/day |
685 |
811 |
96.7 |
0.36 |
786 |
664 |
771 |
771 |
137 |
0.92 |
|
SCN, mg/day |
10.8 |
22.0 |
3.46 |
0.03 |
23.2 |
9.7 |
14.8 |
17.8 |
4.89 |
0.30 |
|
CH4:CO2 |
0.0422 |
0.0357 |
0.0017 |
0.012 |
0.0415 |
0.0355 |
0.0375 |
0.0412 |
0.0024 |
0.25 |
The 100% increase in secretion of thiocyanate in the urine of the goats that consumed bitter as well as sweet cassava foliage (Figure 10) reflected the higher levels of HCN precursors in the leaves of bitter cassava compared with those from the sweet variety (Table 1). Thiocyanate levels in urine appeared to be reduced by the additives with the effects being most pronounced for biochar especially in the goats fed foliage from the mixed sweet-bitter varieties (Figure 11).There were no indications of HCN toxicity. All the goats were healthy (Photo 1).
 |
 |
| Figure 10. Effect of cassava variety on thiocyanate in urine from goats fed only sweet cassava foliage (SW) or with access to sweet and bitter cassava foliage (SW-BI) |
Figure 11. Effect of additives on thiocyanate in urine from goats fed only sweet cassava foliage (SW) or with access to sweet and bitter cassava foliage (SW-BI) |
 |
| Photo 1. A Bach Thao goat from Latin Square 2 (access to both bitter and sweet cassava leaves and petioles) at the end of the experiment showing the excellent body condition |
The methane: carbon dioxide ratio was 16% lower when the goats were fed the mixed sweet and bitter cassava foliage compared with only the sweet variety (Table 4; Figure 12). There was no consistent benefit from the additives -- brewers’ grains or biochar (Figure 13).
 |
 |
| Figure 12.
Ratio of CH4:CO2 in mixed eructed
gas and air from goats fed foliage of sweet or mixed sweet and bitter cassava | Figure 13.
Methane:carbon dioxide ratios in mixed eructed gas and
air in goats offered only sweet cassava foliage (SW) or access to sweet and bitter cassava foliage varieties, in each case with additives of brewers’ grains or biochar or both or none |
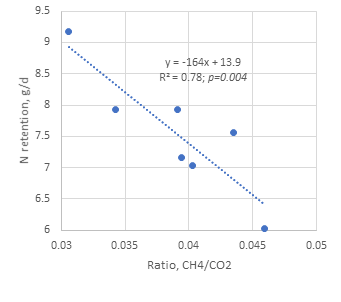
There was a negative relation between the methane: carbon dioxide ratio in mixed eructed gas and air and N retention (Figure 14a). The relationship was particularly strong when an apparent “outlier” result was removed from the data set (Figure 14b).

|
|
|
Figure 14a. Relationship between methane:carbon dioxide ratio in mixed eructed gas and air and nitrogen retention (includes all 8 goats) |
Figure 14b. Relationship between methane:carbon dioxide ratio and nitrogen retention (excluding the outlier result) |
It is proposed that the increases in DM intake and in N retention when the goats had access to foliage of both sweet and bitter cassava varieties are indicative of a superior flow of nutrients to sites of metabolism when the diet comprised equal parts of bitter and sweet cassava compared with feeding only the sweet cassava variety. Condensed tannins are known to facilitate better utilization of dietary protein by forming complexes that make the protein unavailable to rumen microorganisms (Barry and McNabb 1999; Hervas et al 2000; Gerlach et al 2018), thus facilitating its escape from the rumen to the intestines where it is used more efficiently for tissue synthesis (Preston and Leng 1987). However, in the present experiment, tannin levels were similar in both sweet and bitter cassava varieties. A more plausible explanation would appear to be the 72% higher level of cyanogenic glucosides present in the leaves and petioles of the bitter cassava variety which would give rise to HCN when these reached the rumen (Onwuka et al. 1992; Ludgate and Scholz 1992).
It has been reported that HCN is toxic to methanogens (Smith et al. 1985; Cuzin and Labal 1992; Ch Olga Rojas et al 1999; Anachhatra and Amonkuew 2001) but it is possible that they also have a direct inhibitory effect on the overall microbial activity resulting in a shift of digestion to the small intestine, cecum and colon. A shift of this nature was proposed by Phonethiep et al (2016) as the explanation of the observed reduction in rumen methane when the protein component of the diet was of low solubility and therefore with potential to escape undigested from the rumen. Leng (2016, personal communication) proposed this partial digestion shift on the basis of the knowledge that removal of the hydrogen produced by fermentation in these organs was accomplished through acetogenesis rather than by the formation of methane.
Further evidence for the concept of a partial shift in the site of digestion in ruminants fed bitter cassava foliage, is seen: (i) in the outcome of an in vitro rumen incubation (Phanthavong et al 2018) of cassava pulp-urea where it was found that both methane production and mineralization of the substrate DM were reduced when the protein source was leaf meal from a bitter rather than a sweet cassava variety; and (ii) the report of Nguyen Thi Thu Hong et al (2018) that supplementation of the highly digestible vegetable Ipomoea aquatica with Mimosa pigra, a wild leguminous invading shrub, rich in protein and condensed tannins, supported better N retention in goats and reduced more the rumen methane than supplementary foliage from Leucaena leucocepha. It was proposed that the superiority of the Mimosa relative to Leucaena was due to its higher concentration of condensed tannins, facilitating greater rumen bypass of the Mimosa protein and improved energetic efficiency resulting from the associated reduced losses of energy as methane. In this feeding system there was also a positive relationship between the degree of reduction in rumen methane and the increase in N retention.
The inconclusive results for the effects of the additives on methane production are at variance with earlier findings of a reduction in methane production when: (i) brewers’ gains was added at 4-5% of the diet of cattle (Binh et al 2017) and goats (Vor Sina et al 2016; Silivong et al 2018); and when biochar was added at 1% of diets of cattle (Leng et al 2012; Sengsouly and Preston 2016; Vongkhamchanh et al 2018) and goats (Silivong et al 2016; Thuy Hang et al 2018). However, there was a major difference in the design of the present experiment (which was a Latin square changeover with 25-day periods for each of the 4 combinations of additives) and those cited above in (i) and (ii) which were feeding trials with treatments maintained constant during the whole trial period. Assuming that the mode of action of the additives (both brewers’ grains and biochar) is to promote favorable habitats for beneficial microbial communities (Leng 2015), it may be that these effects continue for a considerable time after withdrawal of the additive from the diet. Thus, use of a changeover experimental design is probably counter-indicated for studies with additives that induce carry-over effects on the rumen microbiome.
Anachhatre A P and Amornkaew A 2001 Upflow anaerobic sludge blanket treatment of starch waste water containing cyanide. Water Environment Research, 73(5):622-32.
AOAC 1990 Official Methods of Analysis, 15th edition. Association of the Official Analytical Chemists, Washington D.C.
AOAC 2016 Official Methods of Analysis of AOAC INTERNATIONAL, 20th Edition, Washington D.C.
Barry T N and McNabb W C 1999 The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants. British Journal of Nutrition 81(4):263-72.
Binh P L T, Preston T R, Duong K N and Leng R A 2017 A low concentration (4% in diet dry matter) of brewers’ grains improves the growth rate and reduces thiocyanate excretion of cattle fed cassava pulp-urea and “bitter” cassava foliage. Livestock Research for Rural Development. Volume 29, Article #104. http://www.lrrd.org/lrrd29/5/phuo29104.html
Ch Olga Rojas, Alazard D, Aponte R L and Hidrobo L F 1999 Influence of flow regime on the concentration of cyanide producing anaerobic process cyanide levels during anaerobic digestion of cassava. International Journal of Food Science 27: 329-326.
Cuzin N and Labat M 1992 Reduction of cyanide levels during anaerobic digestion of cassava. International Journal of Food Science 27: 329-326.
Gerlach K, Pries M and Südekum K-H 2018 Effect of condensed tannin supplementation on in vivo nutrient digestibilities and energy values of concentrates in sheep. Small Ruminant Research 161: 57-62.
Hervas G, Frutos P, Serrano E, Mantecon A R and Giraldez F J 2000 Effect of tannic acid on rumen degradation and intestinal digestion of treated soya bean meals in sheep. Journal of Agricultural Science, Cambridge, volume 135, page 305–310
Lehmann J 2007 A handful of carbon. Nature 447 143-144 http://www.css.cornell.edu/faculty/lehmann/publ/Nature%20447,%20143-144,%202007%20Lehmann.pdf
Leng R A, Preston T R and Inthapanya S 2012 Biochar reduces enteric methane and improves growth and feed conversion in local “Yellow” cattle fed cassava root chips and fresh cassava foliage. Livestock Research for Rural Development. Volume 24, Article #199. http://www.lrrd.org/lrrd24/11/leng24199.htm
Leng R A 2017 Biofilm compartmentalisation of the rumen microbiome: modification of fermentation and degradation of dietary toxins. Animal Production Science Review https://doi.org/10.1071/AN17382
Ludgate P and Scholz S (Editors)1992 New Technologies for Small Ruminant Production in Indonesia. Winrock International Institute for Agricultural Development. Morrilton, Arkansas, USA, 68p.
Madsen J, Bjerg B S, Hvelplund T M, Weisbjerg R and Lund P 2010 Methane and carbon dioxide ratio in excreted air for quantification of the methane production from ruminants. Livestock Science 129, 223–227
Minitab 2016 Minitab user's guide. Data analysis and quality tools. Release 13.1 for windows. Minitab Inc., Pennsylvania, USA.
Nguyen Thi Thu Hong, Nguyen Thi Ngoc Trang, Duong Nguyen Khang and Preston T R 2018 Supplementing water spinach (Ipomoea aquatica) with foliage of Mimosa pigra or Leucaena leucocephala and coconut oil; effects on N retention and enteric methane emissions in growing goats; Livestock Research for Rural Development.Volume 30, Article #207. http://www.lrrd.org/lrrd30/12/hong30207.html
Onwuka C F I, Akinsoyinu A O and Tewe O O 1992 Role of sulphur in cyanide detoxification in ruminants. Small Ruminant Research 8(4): 277-284
Phanthavong V, Sangkhom I, Preston T R, Dung D V and Ba N X 2018 Effect of leaves from sweet or bitter cassava and brewers’ grains on methane production in an in vitro rumen incubation of cassava root pulp-urea. Livestock Research for Rural Development. Volume 30, Article #167. http://www.lrrd.org/lrrd30/9/phant30167.html
Phonethep P, Preston T R and Leng R A 2016 Effect on feed intake, digestibility, N retention and methane emissions in goats of supplementing foliages of cassava (Manihot esculenta Crantz) and Tithonia diversifolia with water spinach (Ipomoea aquatica). Livestock Research for Rural Development. Volume 28, Article #72. http://www.lrrd.org/lrrd28/5/phon28072.html
Phuong L T B, Khang D N and Preston T R 2015 Methane production in an in vitro fermentation of cassava pulp with urea was reduced by supplementation with leaves from bitter, as opposed to sweet, varieties of cassava. Livestock Research for Rural Development. Volume 27, Article #162. http://www.lrrd.org/lrrd27/8/phuo27162.html
Preston T R and Leng R A 1987 Matching Livestock Systems to Available Resources in the Tropics and Subtropics. Penambul Books, Australia. Web version http://www.cipav.org/P&L/preston&leng.htm
Preston T R 2015 The role of biochar in farming systems producing food and energy from biomass. In: Geotherapy: Innovative Methods of Soil Fertility Restoration, Carbon Sequestration and Reversing CO2 Increase (Editor: Thomas J Goreau) CRC Press, Tayler and Francis Group, Boca Raton, Florida USA
SAS 2010 Statistical Analysis Software, SAS/STAT | SAS https://www.sas.com/en_us/software/stat.html
Sengsouly P and Preston T R 2016 Effect of rice-wine distillers’ byproduct and biochar on growth performance and methane emissions in local “Yellow” cattle fed ensiled cassava root, urea, cassava foliage and rice straw. Livestock Research for Rural Development. Volume 28, Article #178. http://www.lrrd.org/lrrd28/10/seng28178.html
Silivong P and Preston T R 2016 Supplements of water spinach (Ipomoea aquatica) and biochar improved feed intake, digestibility, N retention and growth performance of goats fed foliage of Bauhinia acuminata as the basal diet. Livestock Research for Rural Development. Volume 28, Article #98. http://www.lrrd.org/lrrd28/5/sili28098.html
Silivong P, Preston T R, Van N H and Hai D T 2018 Brewers’ grains (5% of diet DM) increases the digestibility, nitrogen retention and growth performance of goats fed a basal diet of Bauhinia accuminata and foliage from cassava (Manihot esculenta Crantz) or water spinach (Ipomoea aquatica). Livestock Research for Rural Development. Volume 30, Article #55. http://www.lrrd.org/lrrd30/3/siliv30055.html
Sina V, Preston T R and Tham T H 2017 Brewers’ grains have a synergistic effect on growth rate of goats fed fresh cassava foliage (Manihot esculenta Crantz) as basal diet. Livestock Research for Rural Development. Volume 29, Article #137. http://www.lrrd.org/lrrd29/7/sina29137.html
Smith M R, Lequerica J L and Hart M R 1985 Inhibition of methanogenesis and carbon metabolism in Methanosarcina sp. by cyanide. Journal of Bacteriology, 162, page 67-71.
Thuy Hang L T, Preston T R, Leng R A and Ba N X 2018 Effect of biochar and water spinach on feed intake, digestibility and N-retention in goats fed urea-treated cassava stems. Livestock Research for Rural Development. Volume 30, Article #93. http://www.lrrd.org/lrrd30/5/thuyh30093.html
Vongkhamchanh B, Preston T R, Leng R A, An L V and Hai D T 2018 Effect of biochar on growth performance of local “Yellow” cattle fed ensiled cassava roots, fresh brewers’ grains and rice straw. Livestock Research for Rural Development. Volume 30, Article #168. http://www.lrrd.org/lrrd30/9/bobby30168.html
Received 8 October 2018; Accepted 2 December 2018; Published 1 January 2019