 |
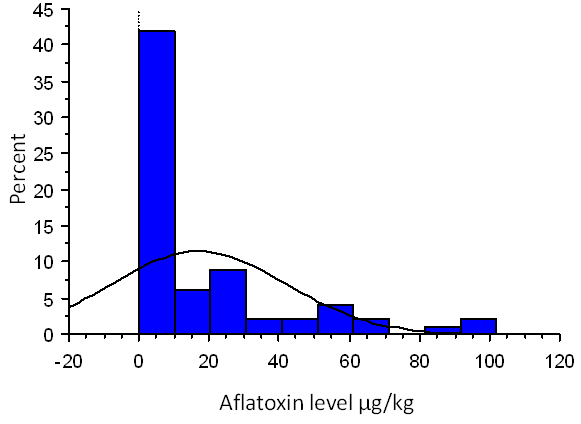
| Figure 1. Distribution of the aflatoxin B1 levels across the concentration range including zero figures |
| Livestock Research for Rural Development 25 (3) 2013 | Guide for preparation of papers | LRRD Newsletter | Citation of this paper |
Contamination of poultry feeds with mycotoxins is one of the major problems associated with the feeding of poultry. Tanzania as any other tropical country experiences a climatic condition that favours growth of fungi and production of mycotoxins in various animal and human feeds. A cross sectional study was conducted in Morogoro Tanzania to assess the level and extent of aflatoxin contamination in commercial poultry feeds in the area. A total of 340 different feed samples including formulated layers and broilers preparations, maize bran and sunflower seedcake were analysed by using indirect ELISA technique. Random sampling was done from animal feed millers, feed sellers and chicken farms.
It was found that 68% (231/340) of all feed samples were contaminated by aflatoxin B1. Minimum contamination frequency was found to be in maize bran 50% while broilers mash had a highest contamination frequency of 91%, and contaminations in sunflower seed cake and layers mash were both 70%. These results gave a significant difference in contamination frequency (p=0.007 at 95% C.I) when broilers mash was compared with the other types of feed. The levels of aflatoxin B1 in the contaminated broilers mash, layers mash, maize bran and sunflower seed cake were 35.8µg/kg, 15.1µg/kg, 9.4µg/kg and 31.6µg/kg respectively and 73% of the contaminated feeds (169/231) exceeded the FAO/WHO level of 5µg/kg. The results indicates that there is a need to build awareness to the feed processors, sellers and farmers on better way to alleviate occurrence of mycotoxins in animal feeds.
Key words: disease, ELISA, fungi, mould, mycotoxins, survey
Mycotoxins are mouldy produced toxins that contaminate a wide variety of food and feeds (Coulombe 1993). At their lifespan, fungi metabolize and produce a broad range from simple to very complex organic compounds that reveal a particular biological activities (Braese et al 2009). Aflatoxin is a term generally used for a group of fungal toxins produced by the fungi Aspergillus species, mainly Aspergillus flavus and Aspergillus parasiticus. These toxins are named from the fungus producing them; that is "A" from the genus name Aspergillus, "fla" from one of the species name flavus added to toxin to give the name aflatoxin (Sweets and Wrather, 2009). There are several different toxins in the aflatoxin group.Mycotoxins contamination of mixed feed and feed ingredients is a worldwide issue and due to ubiquitous nature of them, it is not easy to eliminate them totally from feed and feed ingredients (Azarakhsh et al 2011).Raw materials for compounding food and feeds get contaminated by mouldy and mycotoxin during the pre-harvest (field produced fungi) and/or post-harvest (storage produced fungi) periods (Krnjaja et al 2008).Member species of mouldy fungi that produced during storage include; Aspergillus, Penicillium, Rhizopusand Cladosporium (Maciorowski et al 2007). The natural fungal flora associated with foods is dominated by three genera; Aspergillus, Fusarium, and Penicillium, except for the Fusarium plant pathogens, other natural fungal flora may include commensals as well as pathogens (Murphy et al 2006). Among all species of mouldy fungi, Aspergillus species are the main contaminants of food and feed (Ghiasian and Maghsood 2011).Aspergillus flavus, Aspergillus parasiticus, Aspergillus nomius, Aspergillus tamarii and Aspergillus pseudotamarii mainly they are capable of producing aflatoxins and other mycotoxins, although some other species of genera Penicilliumand Rhizopusare also toxigenic and able to produce mycotoxin (Murphy et al 2006).
Aspergillus species are worldwide distributed probably because they produce numerous airborne conidia which easily spread by air movements and insects (Hedayati et al 2007). There are about 200 species of this mould in the world of which, 16 species of Aspergillus are dangerous to human by causing disease and infection (Dagenais and Keller 2009). This kind of mould grows best in oxygen rich environments and on materials rich in carbon which will provide nutrients they feed on. However some species of Aspergillus can survive in environment with very little nutrients and moisture, just humidity in air this kind are known as Xerophilic (Pettersson and Leong 2011).
The study was carried out in Morogoro municipality (8o00’S 37o00’E / 8oS 37oE) in Tanzania.Feed samples were collected from Morogoro municipality (urban), from different sources including animal feed shops, milling machines, households of chicken keepers and animal feed compounders. Samples were analysed at the Toxicology, Microbiology and Public Health laboratories in the faculty of Veterinary medicine, SokoineUniversity of Agriculture.
A total of 340 feed samples including chick starter, broilers and layers finisher, maize bran and sunflower seed cakes were analysed. These feed samples were collected from 13 animals feed shops, 5 milling factories, 8 chicken keeper households and from 2 animal-feed compounding mills within Morogoro Municipality. For each sample within the category, 1kg feed was collected from the source and thoroughly mixed as have been directed in another study, (Shareef, 2009) to make a composite sample. The number of samples collected for each category is summarized in Table 1 below.
|
Table 1. Number of different sample types analyzed |
|
|
Sample category |
Number of samples |
|
Maize bran |
85 |
|
Sunflower cakes |
85 |
|
Broiler (starter and finisher) |
85 |
|
Layers (starter and finisher) |
85 |
100g of each feed sample was crushed to powder from which extraction solution was made for analysis of aflatoxin B1. The extraction solution was prepared by mixing 20ml of distilled water with 80ml of 100% methanol then swirled completely to mix well. 50g of the ground feed sample and 5.0 g NaCl, were added with 50ml extract solution in a clean blender jar. The mixture was blended for 1 minute in a high speed blender then filtered by Whatman number 41filter paper. 5ml of the filtrate was mixed thoroughly with 20ml of distilled water, re-filtered, stored in sterile bottles and analysed for aflatoxin within 24 hours.
In this study, ELISA analysis was conducted by using ELISA kit PN 53012B from ABRAXIS York city USA.The reagents used were enzyme conjugate (Aflatoxin-HRP enzyme conjugate), calibrators (0, 2, 7.5, 25 and 100 ppb of aflatoxin), antibody solution (Rabbit ant-Aflatoxin antibody), wash buffer, enzyme substrate (Tetramethlbenzidine(TMB)), and stop solution (1NHCl).
50 µl of enzyme conjugate were dispensed into each well of ELISA plate, added with 50 µl of calibrators and samples to the appropriate test wells. Thereafter, 50 µl of antibody solution was added and the mixture was incubated at room temperature for 10 minutes. After incubation the mixture was dispensed, then 200 µl of wash solution was used to rinse each well. Rinsing with wash solution was repeated four times. 100µl of enzyme substrate was added to the wells, incubated for 10 minutes at room temperature, and then 100µl of stop solution were added to each test well. Absorbance was read at 450nm using Multiskan RC plate leader made by ThermoLabsystem, Finland. Concentrations of the standards were plotted against their absorbance on a graph paper. From the straight line that approximately joined the points on the graph, concentrations of the samples were extrapolated from their respective absorbencies.
Aflatoxin B1 concentration in each sample was recorded into Microsoft Excel. Data were calculated as mean ± standard deviation (SD), analyzed using unpaired T-test. Probability of 0.05 or less was considered significant. The statistical package of StartView program version 5.0.1 (SAS institute©1992-1998) was used for analysis and calculations.
The samples were categorized to simplify analysis, where first categorization put the feed samples in the two groups of compounded feeds and non-compounded feeds. Compounded feeds are feeds formulated with different ingredients for different groups of chicken (broiler and layer mash) while non-compounded feeds are crop products that have not been mixed with other ingredients (maize bran and sunflower cake), and which are used as ingredients for making compounded feeds but often given directly to chicken. The second categorization considers the type of the feed sample, where four types namely broiler feeds, layers feeds, maize bran and sunflower seed cake were analyzed. The percentages of contaminated samples in each feed type are shown on Table 2 below. It was found that 68% (231/340) of all feed samples were contaminated by aflatoxin B1. The percentage of contaminated samples was 78.1% for compounded feeds and 60% for non-compounded feeds. In terms of feed types, maize bran recorded the lowest contamination percentage (50%) while highest contamination percentage was recorded in broiler feeds (91.7%) as showed in table 2 below
|
Table 2. ELISA results showing percentage of samples found positive for aflatoxin B1contamintation |
|
|
Feed category |
Percentage of positive samples |
|
Maize bran |
50 |
|
Sunflower cake |
70 |
|
Layer starter and finisher |
70 |
|
Broiler starter and finisher |
91 |
The mean aflatoxin B1 level in all the feeds was 21.5±36.1 µg/kg, where the highest, lowest, and median values for each feed type together with their standard deviation are given in Table 3 below.
|
Table 3. The low, median and highest aflatoxin levels and percentage of samples with aflatoxin level above the Food and Drugs Authority maximum accepted level (20 µg/kg) |
||||
|
Feed type |
Lowest value |
Median value |
Highest value |
% of samples with aflatoxin level > 20 µg/kg |
|
Maize bran |
0 |
1.1 |
64 |
20 |
|
Sunflower cake |
0 |
4.95 |
66 |
25 |
|
Layer starter and finisher |
0 |
5.65 |
82 |
30 |
|
Broiler starter and finisher |
0 |
25.75 |
102 |
67 |
The mean levels for layer feed samples and sunflower seedcake were 15.1±22.2 µg/kg and 31.6±54.3 µg/kg respectively. Intergroup analysis done to compare the aflatoxin B1 contamination levels between the compounded and non-compounded categories by using unpaired t-test at 95% confidence interval couldn’t reveal evidence of significant difference between the two groups (p=0.07). However, in general it was found that the levels were significantly different when analysis for the variance of feed types was conducted by Kruskal-Wallis test (p=0.04). The difference between feed types was due to the high average aflatoxin B1 level in broiler feeds as compared to levels in layer feeds and maize bran (Table 4).
|
Table 4. Comparison of aflatozin B1 level in different sample types |
||
|
Feed category |
Aflatoxin B1 level (µg/kg) |
p-value (compared to broiler feeds) |
|
Broiler feeds |
35.8±35.2 |
|
|
Layers feeds |
15.1±22.2 |
0.04 |
|
Maize bran |
9.4±16.8 |
0.01 |
|
Sunflower seedcake |
31.6±54.3 |
0.06 |
It was found that 169 out of the 231 (73%) samples positive of aflatoxin B1 had levels higher than 5µg/kg higher than the FAO/WHO accepted levels in animal feeds. Distribution of the aflatoxin B1 levels across the concentration range indicates a left skew. Figure 1 below indicates distribution of the levels including zero values while figure 2 indicates distribution of the levels excluding zero values.
 |
| Figure 1. Distribution of the aflatoxin B1 levels across the concentration range including zero figures |
 |
| Figure 2. Distribution of the aflatoxin B1 levels across the concentration range excluding the zero figures |
The findings in this study show that aflatoxin B1 contamination in poultry feeds in Morogoro is 68% in average with variation between different feed types. Several studies have indicated a similar high prevalence of mycotoxins in animal feeds in different places (Lee-Jiuan and Li-Mien, 2006; Shareef, 2009; Alkhalaf et al 2010). The ability of fungi to produce toxic metabolites (mycotoxins) depends not only upon the strain involved but also the condition under which it is grown. For stored grain, toxigenic fungal infection and mycotoxin production results from a complex interaction between moisture, temperature, substrate, oxygen (O2) and carbon dioxide (CO2) concentration and presence of fungal spores (Lakshmikantha, 2011). The optimum condition for Aspegillus species to grow is between temperatures 250C to 300C and relative humidity of 0.90 - 0.99 (Giorni et al 2009). The median temperature range in Morogoro is 25-30°C and relative humidity of 0.8-0.9, proving an ideal environment for fungal growth and mycotoxins production. Occurrence of mycotoxins in feed depends on location as well as environmental condition during harvesting and storage (Osho et al 2007). The aflatoxins occur mostly in tropical regions with high humidity and temperature during crop development when it is damaged by insects, or stressed by heat and drought, and/or after maturation when the crop is exposed to high moisture either before harvest or in storage (Atehnkeng et al 2008; Wild and Yun, 2010.
Broiler feeds were found to be significantly more contaminated with aflatoxinB1 as compared to maize bran (p=0.007) and layer feeds (p=0.049). In practice, broiler feeds are made from different ingredient compositions when compared to layers feeds. Broiler feeds require more amount of energy hence more carbohydrate feeds are used. In that aspect it is common that there is more maize and sunflower cakes incorporated in broiler feeds than in layers feeds. This may have contributed for broilers feeds to be more contaminated by aflatoxin B1 than the other feeds. Despite that maize is postulated to contribute to higher aflatoxin B1 contamination in broiler feeds, maize bran was found to be least contaminated. This can be explained by the fact that most maize bran available as animal feeds is a by product of maize milling in the preparation of flour used for human consumption. In so doing, sorting and cleaning is done and only physically good appearing maize will be used. Sorting and cleaning of maize to remove damaged grains and cobs has been shown to significantly reduce the mycotoxin levels (Sydenham et al1994; Abbas et al 1985). On the other hand, maize used for chicken feeds production are neither sorted nor cleaned and often farmers use the poor quality ones including the damaged maize since they are cheap (Okiki et al 2010). It is also possible that higher contamination in the compounded feeds is due to longer time taken in the food chain, from production, to processing, then compounding to marketing. Prolonged storage of cereals provides more time for multiplication of fungi and accumulation of mycotoxins. Chicken feeds are often compounded from the by-products of human foods like maize, wheat, sunflower, sardines and cotton. The time taken since the by-products are produced to when they are compounded to chicken feeds is probably longer than when the by-product is taken directly before being compounded. Lower aflatoxin B1 in maize bran when compared to sunflower seedcake may be due to it not being stored long due to its high demand as animal feed by farmers keeping animals like cattle, pigs and poultry as compared to sunflower seed cake which is often purchased by poultry keepers only.
Tolerance levels many countries for aflatoxins in foodstuffs and animal feeds are in the range 5 – 50 µg/kg (Hans and Van, 1999). The current United States Food and Drugs Authority (FDA) administration action guideline is 20 µg/kg total aflatoxins in products intended for animal feeds (Schweitzer et al 2001) while for World Health Organization (WHO) 5µg/kg for animals. Tanzania and East Africa Bureau of Standards have set the minimum acceptable levels of total aflatoxins in human feeds at 10 µg/kg (Kaaya and Warren, 2005). The findings from the present study indicate a considerable percentage of animal feeds being contaminated above limits set by different regulatory authorities.
This research was supported by the Belgium-Tanzania Cooperation (BTC) and Newcastle Disease – Avian Influenza control project under generous support from GL-CRSP at UC Davis. The authors are thankful for the support.
Abbas H K, Mirocha C J, Pawlosky R J and Pusch D J 1985 Effect of cleaning, milling, and baking on deoxynivalenol in wheat. Applied and Environmental Microbiology, Volume 50, Article 2, pages 482–486. Retrieved March 21, 2012 from http://aem.asm.org/content/50/2/482.abstract
Alkhalaf A N, Osman K A and SalamaA K 2010 Monitoring of aflatoxins and heavy metals in some poultry feeds. African Journal of Food Science, Volume 4, Article 4, Pages 192-199.Retrived March 21, 2012 from http://www.academicjournals.org/ajfs/pdf/pdf2010/Apr/Alkhalaf%20et%20al.pdf
Atehnkeng J, Ojiambo P S, Ikotun T, Sikora R A, Cotty P J and Bandyopadhayay R 2008 Evaluation of atoxigenic isolates of Aspergillusflavus as potential biocontrol agents for aflatoxinin maize. Food Additives and Contaminants, Volume 25, Article10, Pages 1264 – 1271. Retrieved February 20, 2012 from http://hinari-gw.who.int/whalecomwww.tandfonline.com/whalecom0/loi/tfac19
Azarakhsh Y, Sabokbar A and Bayat M 2011 The frequency of the Most Potentially Toxigenic Fungi in Broiler Feed in Kermanshah Province, West of Iran. American-Eurasian Journal of Toxicological Sciences, Volume 3, Article1, Pages 11-16. Retrieved April 03, 2012 from http://www.idosi.org/aejts/3(1)11/3.pdf
Braese S, Encinas A, Keck J and Nising F C 2007 Chemistry and Biology of Mycotoxins Coulombe A R 1993 Symposium: Biological Action of Mycotoxins. Journal of Dairy Science, volume 76, pages 880-891.
Dagenais R T and Keller P N 2009 Pathogenesis of Aspergillus fumigates in Invasive Aspergillosis. Clinical Microbiology Review, Volume 22, Article 3, Pages 447-465. Retrieved June 15 2012 from http://cmr.asm.org/content/22/3/447.short
D'Mello F J, Macdonald C A, Postel D, Dijksma P W, Dujardin A and Placinta M C 1998 Pesticide use and mycotoxin production in Fusarium and Aspergillusphytopathogens. European Journal of Plant Pathology, Volume 104, Article 8, Pages 741-751. Retrieved June 21, 2012 from http://www.researchgate.net/publication/226935371
Ghiasian A S and Maghsood H A 2011 Occurrence of Aflatoxigenic fungi in cow feeds during the summer and winter season in Hamadan, Iran. African Journal of Microbiology Research, Volume 5, Article 5, pages 516-521. Retrieved September 2012 from http://www.academicjournals.org/ajmr
Giorni P, Magan N and Battilani P 2009 Environmental factors modify carbon nutritional patterns and niche overlap between Aspergillusflavus and Fusariumverticillioides strains from maize. International Journal of Food Microbiology, Volume 130, Pages 213–218.Retrieved August 23, 2012 from https://dspace.lib.cranfield.ac.uk/bitstream/1826/3347/3/niche_overlap_between_Aspergillus_flavus_and_Fusarium_verticillioides-2009.pdf
Hans P and Van E 1999 Worldwide Regulations for Mycotoxins. Third Joint FAO/WHO/UNEP International Conference on Mycotoxins, Tunis, Tunisia, 3-6 March, 1999, Page 7.
Hedayati T M, Pasqualotto C A, Warn A P, Bowyer P and Denning W D 2007 Aspergillusflavus: human pathogen, allergen and mycotoxin producer. Microbiology, Volume 153, Pages 1677–1692. Retrieved September 2012 from http://mic.sgmjournals.org/content/153/6/1677.full
Kaaya N A and Warren L H 2005 A review of past and present Research on Aflatoxin in Uganda. African Journal of Food, Agriculture, Nutrition and Development Volume 5, Article 1, Pages 1-18. Retrieved July 2012 from http://www.bioline.org.br/request?nd05010
Krnjaja V, Stojanovic L, Cmiljanic R, Trenkovski S and Tomasevic D 2008 The presence of potentially Toxigenic Fungi in Poultry Feed. Biotechnology in Animal Husbandry Volume 24, Article 5, Pages 87 – 93. Retrieved October, 2012 from http://www.europeana.eu/portal/record/92040/C74F715BE49477C0AD4FA91F756797836156EA82.html
Lakshmikantha K C 2011 Poisonous compounds can cause serious health effects, impact economy. [http://www.world-grain.com] Site visited on March 28, 2012
Lee-Jiuan C and Li-Mien T 2006 High occurrence of mycotoxins in Asian feedstuffs. World poultry, Volume 10, Article 4 Pages 13 -16.
Maciorowski K G, Herrera P, Jones F T, Pillai S D and Ricke S C 2007 Effects on poultry and livestock of feed contamination with bacteria and fungi. Animal Feed Science and Technology, Volume 133, Pages 109-136. Retrieved March 2012 from http://cat.inist.fr/?aModele=afficheN&cpsidt=18455123
Murphy P A, Hendrich S, Landgren C and Bryant C M 2006 Food Mycotoxins: An update. Journal of Food Science, Volume 71, Pages 51-65. Retrieved June 12, 2012 from http://www.foodtechnology.org/Knowledge-Center/Read-IFT-Publications
Okiki A P, Ojeizeh I T and Ogbimi O A 2010 Effects of Feeding Diet Rich in Mycotoxins on the Health and Growth Performances of Broiler Chicken. International Journal of Poultry Science, Volume 9, Article 12, Pages 1136-1139. Retrieved October 2012 from http://www.pjbs.org/ijps/fin1760.pdf
Osho B, Awoniyi A M T and Adebayo I A 2007 Mycological investigation of compounded poultry feeds used in poultry farms in southwest Nigeria. African Journal of Biotechnology, Volume 6, Article 15, Pages 1833-1836. Retrieved March 23, 2012 from http://www.academicjournals.org/AJb/abstracts/abs2007/6Aug/Osho%20et%20al.htm
Pettersson V O and Leong L S 2011 Fungal Xerophiles (Osmophiles).http://www.els.net. Site visited on March 28, 2012
Schweitzer H S, Quist F C, Grimes L G and Forster L D 2001 Aflatoxin levels in corn available as wild Turkey feed in Georgia. Journal of Wildlife Diseases, Volume 37, Article 3, Pages 657–659. Retrieved April 02, 2012 from http://www.jwildlifedis.org/content/37/3/657.full.pdf
Shareef M A 2010 Mouldy and mycotoxin in poultry feeds from farms of potential mycotoxicosis. Iraqi Journal of Veterinary Sciences Volume 24, Article 1 Pages 17-25.Retrieved March 2012 from http://vetmedmosul.org/ijvs/media/10-1-4e.pdf
Sweets E L and WratherA J 2009 Aflatoxin in corn.http://aes.missouri.edu/delta/croppest/aflacorn.stm. Sited on February 20, 2012
Sydenham E W, Van der Westhuizen L, Stockenstrom S, Shepard G S and Thiel P G 1994 Fumonisin-contaminated maize: physical treatment for the partial decontamination of bulk shipments. Food Additives and Contaminants, Volume 11, Pages 25–32. Retrieved August 12, 2012 from http://www.tandfonline.com/doi/abs/10.1080/02652039409374199
Wild P C and Yun Y G 2010 Mycotoxin and human disease: a largely ignored global health issue. Carcinogenesis oxford journals, Volume 31, Article 1, Pages 71-82. Retrieved October 15, 2012 http://carcin.oxfordjournals.org/content/31/1/71.full
Received 18 December 2012; Accepted 1 February 2013; Published 1 March 2013